CYCLOSPORA
FULLY AUTOMATED RHEONIX C. CAYETANENSIS™ ASSAY
- Fully automated, walkaway detection
- Detects low levels of Cyclospora cayetanensis in produce samples
- Saves four hours of hands-on time per 24-sample run1
- Developed through a Research Collaboration Agreement with the FDA
ASSAY DEVELOPED AND VERIFIED THROUGH U.S. FDA – RHEONIX RESEARCH COLLABORATION
In February 2023 the FDA entered into a research collaboration agreement with Rheonix to develop a fully automated screening assay for the detection of C. cayetanensis in fresh produce, soil and surface agricultural water. The development was completed and announced in June 2023. The new detection method will allow for low levels of the parasite to be detected in a shorter amount of time.1
The FDA-Rheonix collaboration team published verification data in the open access journal Microorganisms:
Development and Evaluation/Verification of a Fully Automated Test Platform for the Rapid Detection of Cyclospora cayetanensis in Produce Matrices.
Hui Zhu1, Beum Jun Kim1, Gwendolyn Spizz1, Derek Rothrock1, Rubina Yasmin1, Joseph Arida2,3, John Grocholl2, Richard Montagna1, Brooke Schwartz1, Socrates Trujillo2, and Sonia Almeria2*Conclusions: The automated Rheonix C. cayetanensis Assay achieved equivalent performance characteristics as the original assay, including the same performance for both inclusion and exclusion panels, and it was able to detect as low as 5 C. cayetanensis oocysts in fresh produce, while significantly reducing hands-on time. We expect that the streamlined assay can be used as a tool for outbreak and/or surveillance activities to detect the presence of C. cayetanensis in produce samples.
1. Rheonix, Inc., Ithaca, NY 14850, USA
2. Division of Virulence Assessment, Office of Applied Research and Safety Assessment, Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Laurel, MD 20708, USA
3. Joint Institute for Food Safety and Applied Nutrition, University of Maryland, College Park, MD 20742, USA
* Author to whom correspondence should be addressed.
1. Source: FDA Cyclospora Prevention, Response and Research Action Plan
Key Publications
FULLY AUTOMATED, WALK-AWAY DETECTION OF C. CAYETANENSIS IN PRODUCE

ASSAY PERFORMANCE FEATURES1
- Sensitive and specific detection of C. cayetanensis in a range of produce samples
- Detects as low as 5 oocysts in fresh produce
- Specificity and sensitivity comparable to FDA-developed method
BENEFITS
- Runs on fully automated Rheonix Encompass Optimum™ workstation
- Reduced hands-on time2
- Less equipment = reduced costs
 2. Compared with prior workflow
2. Compared with prior workflow
WHAT IS CYCLOSPORIASIS AND WHY IS IT A PROBLEM?
Cyclospora cayetanensis (C. cayetanensis) is a protozoan parasite that causes cyclosporiasis and is spread by people ingesting contaminated food or water. While U.S. cases were initially linked to imported fresh food and travel, investigations of an outbreak in 2018 revealed that domestic produce was also at risk.
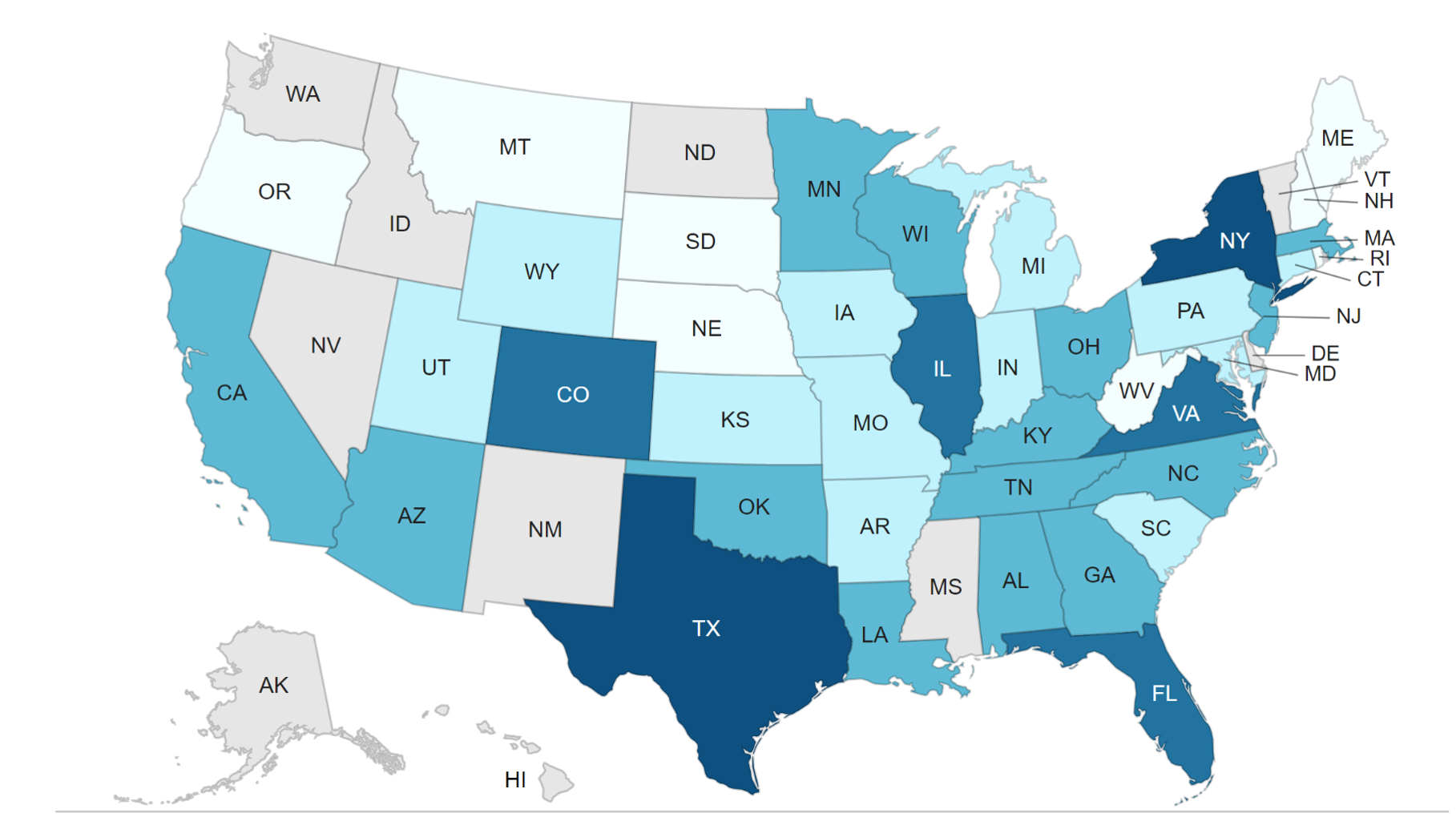
Over 10,000 cases of U.S. domestically acquired cyclosporiasis1 have been reported since the 2018 incident; these outbreaks have been associated with fresh produce consumption, including herbs, berries, and leafy greens.
Domestically Acquired Cases of Cyclosporiasis:
41 jurisdictions, including 40 states and New York City, have reported a total of 2,272 laboratory-confirmed cases of cyclosporiasis.